What Best Describes Quaternary Structure of Proteins
Small usually hard proteins with a rigid structure medium and large usually soft proteins with a flexible structure. Proteins can be divided into three sub-categories.
Protein Structure Introduction To Chemistry
The quaternary structure describes the arrangements of subunits in a protein that contains more than one subunit.

. -secondary structure is maintained by hydrogen bonding-tertiary structure is another level of folding and compacting-arises from addition hydrogen bonds van der Waals forces and disulfide bonds-the result is a complex three-dimensional protein that is now in most cases functional-quaternary structure is the most complex structure. Ø This is the reason for the denaturation of proteins in the acidic or basic medium. This structure is formed as a result of the linkage of polypeptide chains with each other.
Dimers were the most common representing. The promoter is a nontranscribed region of a gene. Quaternary Structure- The aggregation of multiple polypeptide subunits.
There are only 17 amino acids found in Martian proteins. SDS denatures secondary tertiary and quaternary structures by binding to hydrophobic protein regions and it confers a net negative charge on the proteins which results in a constant charge-to-mass ratio. Two of which are α α 1 α 2 and the other two are β β 1 β 2.
For a detailed description see Biological Assemblies on PDB-101. Thus these proteins show structure-function relationship. The Quaternary structure of hemoglobin describes that it is made up of four polypeptide chains.
34 Quaternary structure. The biological assembly reported in the SMTL is retrieved from the PDB entry. A covalent bond formed from two thiol.
Four major types of attractive interactions determine the shape and stability of the folded protein. The comparison of the size of an exemplary metal-based nanostructure gold nanocluster with a diameter of 14 nm and different proteins can be found in Fig. Ø Ionic bonds are weak bonds and they are very fragile in an aqueous medium.
Ø Tertiary and quaternary structures of proteins are stabilized by ionic bonds. Secretion of proteins into the periplasmic space has been the traditional approach for producing oxidized proteins in vivo and is well suited for proteins that are toxic to the cell when expressed in the cytoplasm Cornelis 2000. Include SDS in the sample buffer.
The highest two peaks in the rotation search have correlation coefficients of 231 and 205 and are related by a rotation of 180. Coli genome only about 20 were expected to be monomeric. In a recent evaluation of the proteins encoded by the E.
Which of the following statements are true regarding quaternary structure of proteins Refers to organisation and spatial arrangements of amino acids within a polypeptide chain Refers to. Quaternary structure describes the arrangement of the polypeptide chains in the multi subunit arrangement. Ionic bonding hydrogen bonding disulfide linkages and dispersion forces.
The next highest peak is considerably weaker at 88. For the quaternary enzyme complex the structure was solved using a monomer of the refined quinternary complex as a the search model in MR. Singular amino acids are the building blocks of life and can be linked to form oligopeptides polypeptides and proteins inside the cellThis occurs during a process called protein synthesisVarying sequences of the.
Following translation translocation or insertion into ER membrane proteins are modified to assume their final structure and therefore function. The remainder are homo- or hetero-oligomers or macromolecular assemblies or polymers. In unit 610 there is an example of the production of antibody fragments by independent secretion of heavy chain.
Which of the following statements best describes the promoter of a protein-coding gene. The most common amino acid in beta bend is cysteine glycine serine methionine 10. Many proteins are made up of multiple polypeptide chains often referred to.
Primary structure of protein determines the tertiary structure All of these 9. Some proteins a minority are composed only of a single polypeptide chain. The biological assembly biounit describes the oligomeric state or quaternary assembly which is thought of as the biologically most relevant form of the molecule.
Ø Even a change in the pH may breakdown the ionic bonds. SDS binds proteins in. This describes proteins which consist of two or more chains of polypeptides.
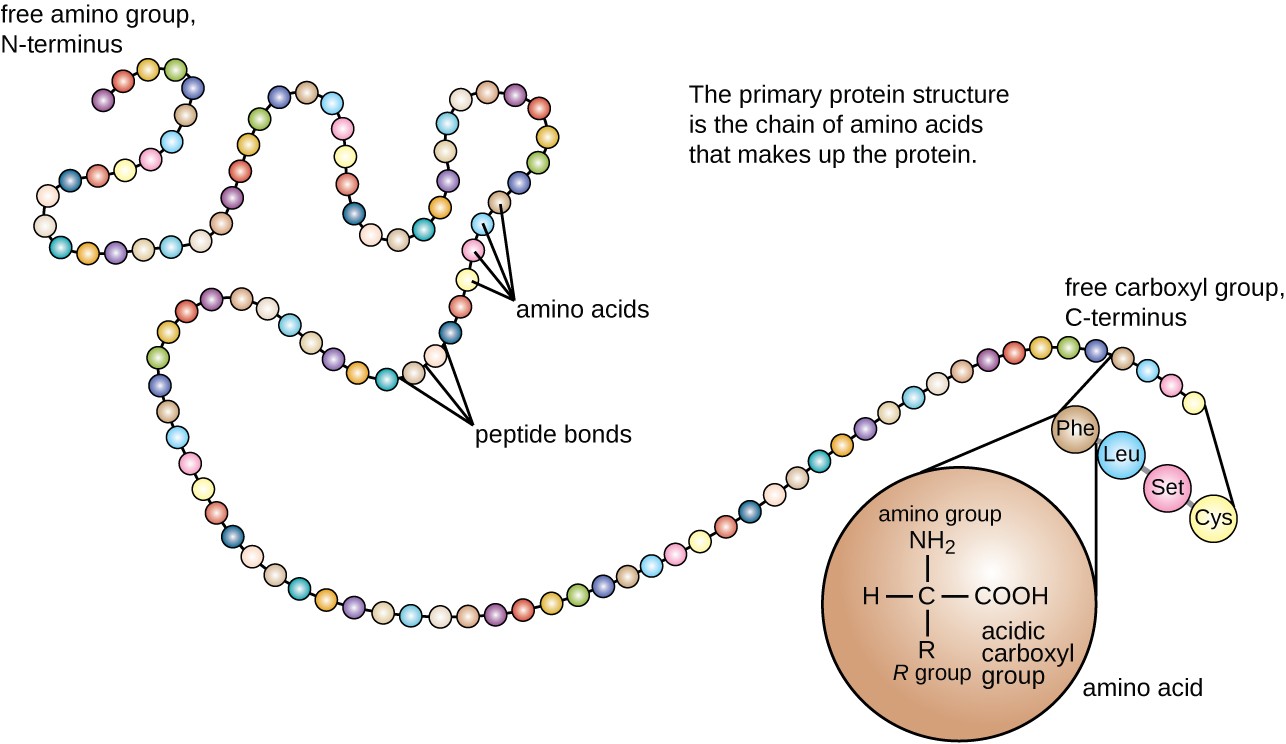
The polypeptide definition describes a chain of more than twenty and less than fifty amino acids bound together via covalent peptide bonds. The two alpha chains are opposite to each other and adjacent to each β-chain. In the views of above in this chapter we have described briefly from amino acids the building block of proteins to quaternary structure.
What Is The Functional Importance Of Proteins At The Quaternary Structure Quora

Quaternary Structure An Overview Sciencedirect Topics

Primary Secondary Tertiary And Quaternary Structures Of Proteins Youtube
Comments
Post a Comment